
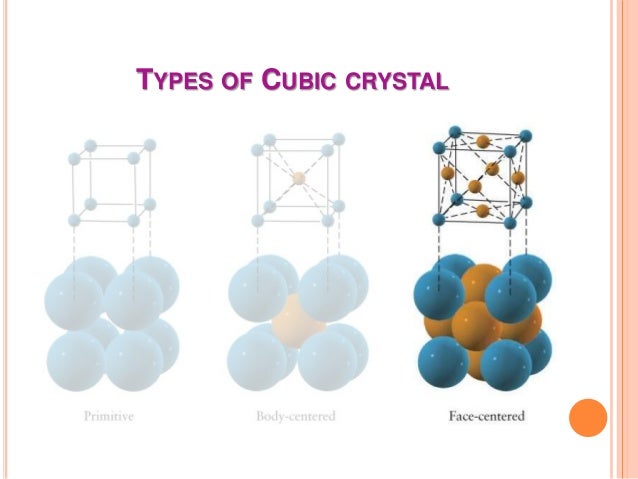
Where n n n is the number of atoms per unitcell and for BCC n n n = 2.0, V S V_\\Ī PF = V C n V S = ( 3 4 R ) 3 2.0 ( 3 4 π R 3 ) = ( 3 4 ) 3 R 3 2.0 ( 3 4 π R 3 ) = 0. SolutionĪPF is given by equation (3.3) in the form For an atom to make such a move the following two conditions must be met. APF is basically the fraction of atoms to void. Pin By Charlie Bravo On Ingenierias Unit Cell The Unit Cell Electrical magnetic and dielectric properties. In the crystal structure, atomic packing factor (APF) or packing efficiency or packing fraction is the volume of atoms in a unit cell divided by the volume of the unit cell.We are asked to prove that the atomic packing factor APF for BCC is 0.68. BCC has 2 atoms per unit cell lattice constant a 4R3 Coordination number CN 8 and Atomic Packing Factor APF 68. In BCC (body-centered cubic) crystal, the arrangement will be as follows.corresponding to the well-known body centered cubic (bcc) structure.
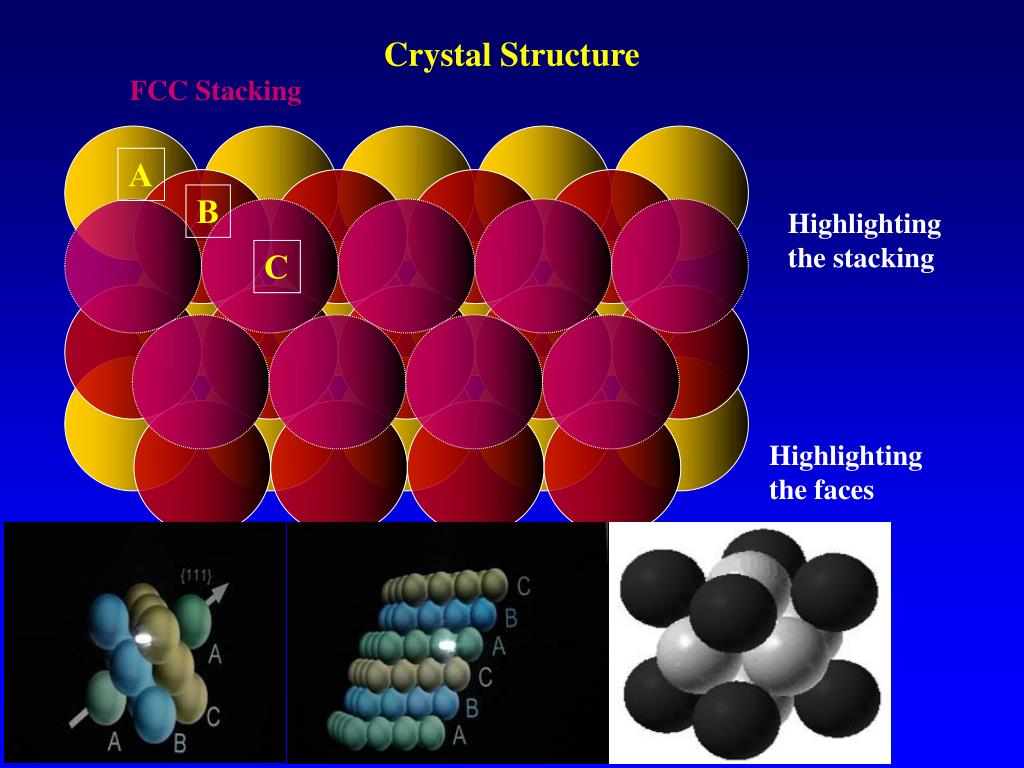 The unit cell is the smallest portion of a crystal lattice which, when repeated in different directions, generates the entire lattice. The packing of atoms in various crystal structures is a.
The unit cell is the smallest portion of a crystal lattice which, when repeated in different directions, generates the entire lattice. The packing of atoms in various crystal structures is a. 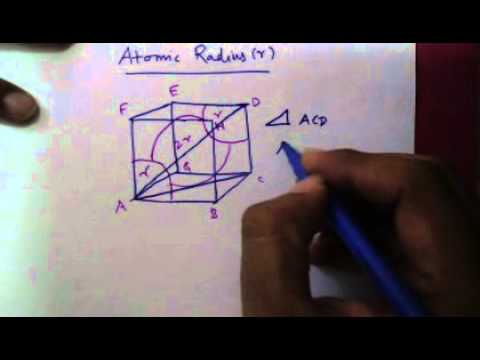
Thus, a regular three-dimensional arrangement of points in space is called a crystal lattice. Question: What Is The Atomic Packing Factor What Are The Atomic Packing Factors Of BCC, FCC And HCP Does The Volume Increase Or Decrease When FCC Iron Changes To BCC Iron Why This problem has been solved See the answer What is the atomic packing factor What are the atomic packing. If the three-dimensional arrangement of constituent particles in a crystal is represented diagrammatically, in which each particle is depicted as a point, the arrangement is called a crystal lattice. Hence the atomic packing factor for a material with body centred cubic structure is ((3)a3)/8a3 0. where a is side of the cube and r is atomic radius. The main characteristic of crystalline solids is a regular and repeating pattern of constituent particles. In addition to the above two types of arrangements a third type of arrangement found in metals is body centred cubic (bcc) in which space occupied is about 68. In the crystal structure, atomic packing factor is the volume of atoms in a unit cell divided by the volume of the unit cell.







 0 kommentar(er)
0 kommentar(er)
